21 January 2026

Clinical stage oncology company Prescient Therapeutics (ASX: PTX) presented exciting new data on their OmniCAR and CellPryme cell therapy platforms at the prestigious International Society of Cell & Gene Therapy (ISCT) in Paris earlier this month. The company is at the forefront of solving the critical challenges facing one of the world’s most exciting next-generation cancer therapies, CAR-T. OmniCAR has demonstrated a clear ability to drastically enhance CAR-T tumour killing abilities in glioblastoma and acute myeloid leukaemia.
Clinical stage oncology company Prescient Therapeutics (ASX: PTX) presented exciting new data on their OmniCAR and CellPryme cell therapy platforms at the prestigious International Society of Cell & Gene Therapy (ISCT) in Paris earlier this month. The company is at the forefront of solving the critical challenges facing one of the world’s most exciting next-generation cancer therapies, CAR-T. OmniCAR has demonstrated a clear ability to drastically enhance CAR-T tumour killing abilities in glioblastoma and acute myeloid leukaemia.
Immunotherapies work by harnessing a patient’s immune system to fight cancer. One of the most exciting clinical applications in this field has been Chimeric Antigen Receptors (CAR) -T cell therapy, which changes the genes of T-cells (white blood cells that are part of the immune system) to find and destroy cancer cells.
CAR-T cell therapies are associated with unique complications that can arise, which may limit their effectiveness. Furthermore, conventional CAR-T cell therapies have thus far shown limited efficacy to treat solid tumours, due to factors such as the immunosuppression in the tumour microenvironment (TME), inefficient T cell trafficking and heterogeneity of antigen expression. In the context of both solid and haematological malignancies, relapse is common due to tumour escape.
Image: Prescient Therapeutics.
OmniCAR
Prescient’s OmniCAR platform is aiming to address key challenges of current generation CAR-T therapies by making treatments safer, more effective, more affordable and longer lasting. For example, a key limitation of CAR-T therapy is T-cell associated toxicities. There have been many unfortunate incidents of T-cells going rogue and causing horrific damage to patients. While this is a complex issue to tackle, PTX’s OmniCAR platform gives clinicians unprecedented control over T-cells – allowing oncologists to switch them off once they’re infused to immediately stop treatment.
Critically, OmniCAR can be efficiently armed with at least three different binders at any one time, which is crucially important when targeting cancers with multiple antigens – such as tumours. OmniCAR tumour killing can be precisely controlled by varying the binder dose, which is excitingly reminiscent of the ‘dose response’ seen in conventional medicines – but not currently available in CAR-T.
The OmniCAR universal immune receptor is comprised of two discrete components that, when combined, forms an unbreakable covalent bond to act as an immunologic bridge to target tumour antigens, eliciting an antigen specific T cell response.
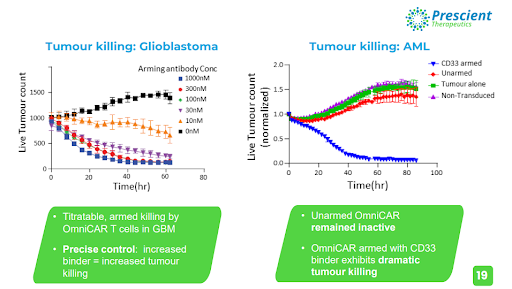
The exemplary utility of OmniCAR is perfectly shown in the graph below, where the unprecedented level of control OmniCAR gives oncologists over their CAR-T therapies has resulted in the near eradication of live tumour cells in glioblastoma and acute myeloid leukaemia.
Image: Prescient Therapeutics.
Glioblastoma
Glioblastoma (GBM) is the most common primary brain tumour in adults and is notorious for its lethality. They are multiforme and grow from a type of glial cell called astrocytes, which are a type of brain cell that support and protect nerve cells (neurons) by providing them with oxygen and nutrients, and removing dead cells. The disease has a poor prognosis, with the average survival time from diagnosis being 12-18 months. Only 25% of patients survive more than one year, and only 5% of patients survive more than five years.
Despite considerable progress in understanding the biology of glioblastoma, there have been no regulatory drug approvals in the United States since 2009. Additionally, from the multitude of phase three clinical trials conducted between 2005-2021, only one study produced positive findings. So far, despite clear efficacy in many other malignancies, immunotherapies have also shown little effectiveness in GBM. A significant contributor to this issue is the limited number of specific antigens in GBM that are homogeneously expressed in the tumour, a problem that recent developments in CAR-T have shown promising potential to solve.
Ideally, target antigens should be homogeneously expressed on tumours without presence in healthy tissue. Two antigens (amongst a few others) worth noting are Epidermal Growth Factor Receptor Variant 3 (EGFRvIII) and Human Epidermal Growth Factor Receptor 2 (HER2). EGFRvIII is a transmembrane receptor tyrosine kinase that is amplified in half of GBM tumours, and is minimally expressed in the central nervous system. HER2 is a receptor tyrosine kinase normally expressed in epidermal tissue at low levels, upregulated in 80% of GBM tumours.
Armed with the HER2 and the EGFRvIII binders, OmniCAR demonstrated its ability to specifically target only the instructed antigen, while being able to switch between antigens or switch off entirely, according to instructions provided by the practitioner.
Effective killing of tumour cells was observed, and a clear correlation between increased dosage of binders being associated with reduced live tumour cells was established, while unarmed (no binder dose given) T cells resulted in a significant increase in live tumour cells. The mixing of two different GBM cells mimics the heterogeneity of the disease in humans, which is a key fundamental principle of cancer treatment being implemented in the new age of immunotherapy research, where targeting only one antigen has not demonstrated adequate efficacy.
Interestingly, allowing the OmniCAR T-cells to lay dormant in vivo for a period of time prior to dosing of binders was associated with potent anti-tumour efficacy. OmniCAR T-cells were rested for three weeks, during which time the tumour size grew in size, before arming with binders was activated which resulted in an immediate and potent tumour killing reaction.
Acute Myeloid Leukaemia (AML)
Acute Myeloid Leukaemia (AML) is the name given to a group of leukemias that develop in the myeloid cell line in the bone marrow. Myeloid cells are red blood cells, platelets and all white blood cells excluding lymphocytes. AML is characterised by an overproduction of immature white blood cells, called myeloblasts or leukemic blasts. There are eight different subtypes of AML, categorised based on the appearance of leukemic cells under the microscope, the type of blood cell involved and at what point it stopped maturing properly in the bone marrow.
The five year survival rate in the US for people over 20 is 29.5%, and treatment typically consists of chemotherapy and stem cell transplantation. Conventional chemotherapy for AML, including induction and consolidation treatment, is only partially effective. Patients often require bone marrow transplantation and multiple rounds of consolidation therapy – to which they are often refractory.
CAR-T has shown excellent results in treating other types of blood cancers, namely B cell lymphomas. Researchers are hoping similar outcomes can be achieved in AML, and are identifying antigens that are expressed in AML.
Most efforts of developing monoclonal antibodies or antibody-drug conjugates (ADCs) for AML have focused on targeting Cluster of Differentiation Antigen 33 (CD33). Leukemic blasts and myeloid leukaemia-initiating cells express CD33, while the antigen does not appear on the surface of primitive stem cells or multipotent progenitor cells – making it a favourable target for immunotherapy.
Armed with CD33, OmniCAR exhibited dramatic tumour killing capabilities, as the live tumour cell count was rapidly diminished over the testing period. Once again, unarmed OmniCAR remained inactive and the tumour cell count increased over the same testing period.
CellPryme
Prescient’s cell therapy enhancement platform CellPryme is working to solve a key problem in CAR-T. Current therapies are making too many effector and effector memory T-cells, when they need to be making more stem and central memory T-cells (Tcm).
CellPryme complements OmniCAR and has two distinct components which can be used seperately, but have distinct synergies when used together.
CellPryme-A is an adjuvant therapy provided to patients that boosts tumour killing and host survival of conventional CAR-T cell therapies. It achieves this by overcoming the tumour’s hostile microenvironment and significantly enhancing the expansion CAR-T cells within the host.
The platform’s benefits are even greater when used in conjunction with PTX’s CellPryme-M, which enhances adoptive cell therapy performance by shifting T and natural killer (NK) cells towards a central memory phenotype. It’s a 24-hour, non-disruptive process during cell manufacturing that improves T cell persistence, and increases their ability to find and penetrate tumours.
Image: Prescient Therapeutics.
In CAR-T therapy, once blood is collected from the patient, T-cells are isolated and genetically altered to have cancer recognising receptors. Millions of these cells are then grown, during which time CellPryme-M can be incorporated into the process with the activated T-cells, which enriches the amount of central memory T-cells (Tcm).
During a recent preclinical model of breast cancer, in conjunction with the Peter MacCallum Cancer Centre, University of Melbourne and Victorian Comprehensive Cancer Centre, CellPryme-M demonstrated a clear boost in Tcm when applied to CD4+ and CD8+ T-cells. CD4+ and CD8+ are the main types of lymphocytes in cell-mediated immunity and play a central role in anti-tumour responses. CellPryme-M also protects the T-cells from exhaustion after repeated challenges by tumour cells, highlighting its ability to retain central memory cells.
The tumour microenvironment (TME) is the complex ecosystem surrounding solid tumours and the origins of blood cancers (eg. bone marrow, spleen, lymph nodes etc). It protects and nurtures the cancer by acting as a ‘force field’ that bluntens the effectiveness of cancer therapies.
CellPryme-A attenuates the TME without compromising the CAR-T cells. It modifies the TME in a way that allows CAR-T cells to access the tumour in order to attack, and it does not impact CAR-T cell stem or memory. The platform also reduces the amount of terminally exhausted CAR-T cells in the tumour.
An in vivo study using colon cancer cells that created a 140mm2 tumour showed that treatment with CellPryme-A and CellPryme-M CAR-T cells reduced the size of the tumour to just 12mm2. This was compared to 50mm2 with just CellPryme-A and CAR-T cells, and two tumours of 53mm2 and 133mm2 in size, respectively, with CAR-T cells alone.
Many shots on goal
These developments with Prescient’s OmniCAR and CellPryme platforms come at a gripping time for the biotech company. On top of the cell therapy platforms, Prescient has two personalised therapies, PTX-100 and PTX-200 which are showing encouraging activity in areas of unmet need. The company has expanded the current PTX-100 Phase 1b trial in preparation for a potential Phase 2 trial. Stellar Phase 1b trial results showed a wealth of positive data – including two complete responses in patients with relapsed and refractory (r/r) Peripheral T-cell Lymphoma (PTCL).
Prescient Therapeutics held a special cell therapy briefing last week, where they summarised the data presented at the ISCT conference. Click here to watch a recording of the session.
To stay up to date with Prescient’s news and announcements, register your details on their investor portal.
Reach Corporate provides Corporate Advisory Services, including managing investor communications on behalf of Prescient Therapeutics Limited and may receive fees for its services.
Past performance is not a reliable indicator of future performance.