21 January 2026

When JVC launched VHS videos in 1971, it started a consumer revolution that permanently altered the way the public consume media.
When JVC launched VHS videos in 1971, it started a consumer revolution that permanently altered the way the public consume media.
But in the 44 years that followed, consumers have shifted from VHS, to DVD, to Bluray, to finally abandon physical media altogether for streaming services in the past decade.
Now VHS is remembered only as the lumbering first steps to something greater; and the same will soon be said of CAR-T cancer treatments, according to Prescient Therapeutics CEO Steven Yatomi-Clarke.
Mr Yatomi-Clarke was recently featured in the Australian Financial Review, where he shared his thoughts on the progress of this new breed of biotech companies escalating the fight against cancer. You can read the article here. Please note the article is only available to paying AFR subscribers however we have summarised the highlights below for your convenience.
“As amazing as CAR-T is now, it’s like VHS,” he said.
“That technology revolutionised how people watched movies, but who uses that today? In 10 years we’ll look back and this will be as bad and as expensive as CAR-T will ever get.”
Source: Australian Financial Review
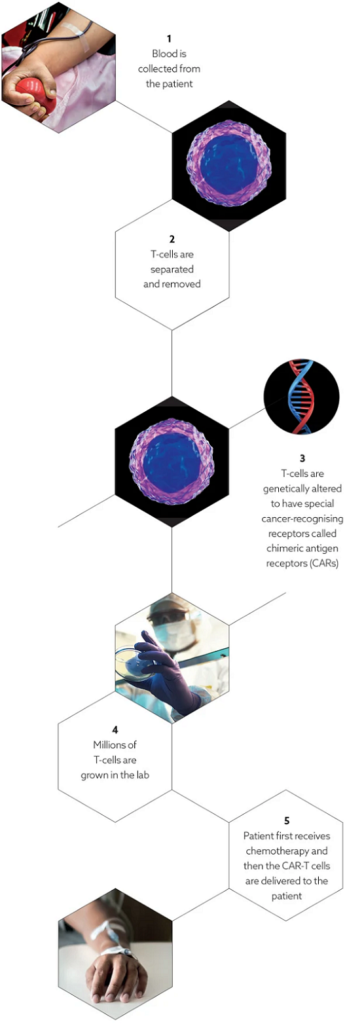
Current generation CAR-T therapies have had great early success in certain blood cancers, but are plagued with safety issues; the inability to control living cells once they are infused into patients; and considerable limitations on the cancer targets it can recognise and attack. In addition, the time and cost of a bespoke treatment is considerable. All of these limitations are preventing the application of CAR-T in other cancers.
This is where Mr Yatomi-Clarke and his team at Prescient have focused their energies: on building the next generation of CAR-T treatments, through their OmniCAR platform.
OmniCAR is based on technology licensed from Oxford University and the University of Pennsylvania – a globally-acknowledged pioneer and leader in CAR-T.
The OmniCAR platform is creating the next generation of CAR-T therapies that are safer, controllable and able to be directed against a myriad of different cancers. It seeks to give clinicians greater control over the activity of the CAR-T cells infused into patients, meaning the cells can be redirected against different tumors.
OmniCAR even gives clinicians the ability to switch the modified cells off if they’re triggering a cytokine storm (where the immune system escalates into a feedback loop of inflammation, with potentially lethal consequences).
Furthermore, OmniCAR can be used to create ‘off-the-shelf’ CAR-T cells, which would be key in overcoming the burdensome cost and logistics endured by current bespoke approaches.
So while Prescient isn’t the first to bring CAR-T treatments to the market, Mr Yatomi-Clarke is comfortable that those companies with current generation CAR-T have paved the way for next generation CAR-T, and is confident that “the second or third mouse will get the cheese”.
“(CAR-T) is not like normal drug development. It’s like an infrastructure play to harvest the cells, engineer them and expand them,” he told the AFR.
“I know whoever can solve some of the safety and flexibility aspects could bring this therapy to many, many more patients.”
Mr Yatomi-Clarke believes the next decade will see the development of ‘off-the-shelf’ CAR-T drugs, and added improvements in cost and safety will mean this type of treatment will start to be used for other diseases.
“Currently we can’t begin to justify the risk reward,” he said. But OmniCAR can help change that.
Prescient Therapeutics is pursuing three programs for its internal OmniCAR development, including acute myeloid leukaemia, solid tumours such as breast, ovarian and gastric cancers, and glioblastoma.
CAR-T poised to be ‘fourth pillar’ of cancer treatment
Professor Phillip Darcy, senior research fellow and group leader at the Peter MacCallum Cancer Centre in Melbourne, and member of Prescient’s Scientific Advisory Board, said he’s “more optimistic than ever” about the future of CAR-T.
“In such a short time it’s gone from an idea on a lab bench into patients, and it works,” he told the AFR.
“In 10 to 15 years immunotherapy has become the fourth pillar of treatment for patients and it’s a wonderful advancement.”
Professor Darcy conducted a clinical CAR-T trial on patients with acute myeloid leukaemia (representing a first in Australia), and he is currently working on a separate trial using CAR-T in non-small cell lung carcinoma patients.
And the risks once associated with CAR-T have even begun to diminish in the past three years, as clinicians become more attune to the side effects, he said.
Billions flow into industry
The CAR-T industry currently consists of at least 30 businesses globally looking to develop these treatments and billions of dollars being invested into the sector.
And developers of these drugs have become hot property, with Gilead Sciences purchasing Kite Pharma for $US11.9 billion in 2017.
Celgene then bought Juno Therapeutics for $US9 billion in 2018, before Bristol-Myers Squibb purchased Celgene for $US74 billion in 2019.
Kite Pharma is one of only two businesses with CAR-T drugs in the market (Yescarta and Tescartus). The other company is Novartis, which manufactures Kymriah.
In February, Novartis received approval from the Therapeutics Goods Administration for Cell Therapies (based in the Peter MacCallum Cancer Centre) to begin producing and supplying Kymriah for eligible patients.
AFR subscribers can read the full article including Mr Yatomi-Clarke’s discussion on CAR-T by clicking here.
If you would like to stay updated on all future announcements or receive invites to upcoming company investor briefings, please update your details on Prescient’s investor centre.
Reach Markets who have been engaged to assist PTX with investor communications.
Sources: